See other Health Articles
Title: Utilization of Placebo Rat Poison in Clinical Trials: Raising the Bar from Sugar Pill to Rodenticide
Source:
The Bonkers Institute
URL Source: http://www.bonkersinstitute.org/rat.html
Published: Jun 25, 2010
Author: Isaac Bonkers, M.D., Principal Investiga
Post Date: 2010-06-25 15:57:06 by Original_Intent
Keywords: bonkers, FDA, drugs, fraud
Views: 179
Comments: 15
Utilization of Placebo Rat Poison in Clinical Trials: Raising the Bar from Sugar Pill to Rodenticide Methodius Isaac Bonkers, M.D., Principal Investigator Before obtaining FDA approval to market a new drug, the manufacturer must provide evidence demonstrating not only safety, but also efficacy and superiority to placebo. Such evidence is commonly acquired through randomized, double-blind, placebo-controlled clinical trials, long considered the gold standard in biomedical research. This article will focus on the use of placebos in controlled trials and offer a modest proposal for improving their clinical utility and statistical relevance, clinical relevance and statistical utility, relevant statistical utility in a clinical setting, and so on. The general public has come to expect that any drug approved by the FDA will work better than a placebo sugar pill. This expectation seems perfectly fair, reasonable, and not too much to ask, but the average citizen lacks the sophistication to understand the heavy burden such an expectation places on the pharmaceutical industry, diverting scarce resources away from advertising, promotion and public relations into less cost-effective sectors such as laboratory testing, analysis and record-keeping. Proving that a new drug works better than a sugar pill is much harder than it sounds, often forcing the manufacturer to conduct one study after another in an attempt to achieve positive results. A study showing negative results is absolutely worthless to a drugmaker hoping to market a new product, and must be abandoned at great expense. A new study must then be initiated at further expense, with no guarantee of success even after considerable tweaking and massaging of data in remarkably creative ways. To modernize an outdated and cumbersome drug testing and approval process, conventional trial protocols and methodology must be re-examined, and various modifications need to be considered. One such modification would be the immediate elimination of placebo sugar pills in all clinical trials, to be replaced instead with placebo rat poison. This proposal is based on solid medical evidence, readily justified and easily explained. Clinical researchers have long recognized the superior value of an active placebo, designed to mimic the side effects of a tested drug, over the questionable value of an inactive, inert placebo with negligible side effects. Compared to the minimal side effects of a sugar pill, rat poison's side effects more closely resemble a typical prescription drug. Patients administered rat poison would be more apt to believe that any subsequent headache, nausea, diarrhea, fatigue, and dizziness were caused by medication, and less likely to suspect placebo. The same is true of physicians conducting the study, who would find it harder to distinguish a tested drug from rat poison than it is to tell the difference between a potent new groundbreaking drug and a dummy sugar pill. In this way, the use of rat poison would help ensure the integrity of a clinical trial's double-blind feature. Moral, Ethical and Medical Considerations Objections might be raised to the use of rat poison due to moral, ethical, or medical concerns. Such concerns are not grounded in science, arising from misconceptions about the origin and nature of various poisons and their comparative toxicity relative to the latest advances in biopharmacology and modern medicine in general. One of the most common rodenticides is warfarin, a chemical compound introduced commercially in 1948 and still widely used as rat poison today, sold under brand names like Adios, Dethnel, Kaput, Kumatox, Rat-B-Gone and Warfarat. Scientists discovered that small doses of warfarin, though lethal to laboratory mice, could actually be of therapeutic value to humans. In 1954 the FDA approved warfarin as a human anticoagulant, and today it remains the anticoagulant of choice, commonly prescribed for the prevention of blood clots in patients with a history of heart disease. Brand names include Coumadin and Jantoven. Serious life-threatening side effects are relatively rare in most patients, especially at low doses of 1 milligram or less. fda sleeps while common medications poison the elderly Warfarin is colorless and tasteless, two qualities which are highly desirable in a placebo. If administered at a very low dose of 0.25 mg in a controlled trial lasting 8 weeks or less, warfarin's toxicity would almost certainly remain within reasonably acceptable limits in healthy patients who had been carefully screened for pre-existing medical conditions after duly signing consent forms and standard legal disclaimers strictly limiting the liability of any individual or corporation responsible for conducting, supervising and/or sponsoring said trial. In most clinical trials, any risk posed to a patient by placebo rat poison would be far smaller than risks associated with a tested drug, especially if the drug being tested were a psychotropic substance targeting neurotransmitters within the basal ganglia, thalamus, hippocampus or any other region of the brain. Stated simply, a tiny amount of rat poison that has the effect of slightly thinning the blood would carry significantly less risk than a psychoactive compound that alters the brain -- the single most complicated, intricately-structured object in the known universe, the least understood of all organs in the human body and, according to some sources, the very seat of the soul. Profound implications for drug marketing and advertising Replacing sugar pills with rat poison would have far-reaching implications for the way new drugs are marketed to the public. Claiming that a drug works "better than a sugar pill" is simply another way of saying it's better than nothing. On the other hand, saying a drug is "better than rat poison" may cause patients to think twice before swallowing it. Describing adverse effects as "similar to rat poison," "not as bad as rat poison," or "milder than rat poison" would be a simple yet effective reminder to both patients and physicians that FDA-approved prescription medicines are seldom fatal when taken as directed. Perhaps the day is not far off when pharmaceutical companies will be able to reap millions of dollars in profits without investing a single dime in research and development. Great strides are being made in this direction. One can only marvel at the growing list of super best-selling blockbuster drugs which have proven themselves, time and again, to offer little if any benefit over simple sugar pills, and to be neither safer nor more effective than older drugs whose patents have expired. This is certainly true of "new generation" psychiatric drugs such as antidepressants and antipsychotics, which are among the most expensive and widely prescribed of all medications on the market today, despite a track record demonstrating their absolute inability to cure anything whatsoever. Creating a perceived need for lifelong treatment with expensive drugs that cure nothing is one of the most astounding achievements of the modern medical era, yet recent advances in pharmaceutical marketing and merchandising have also come with a downside. Potent drugs with unknown long-term consequences, toxic side effects, and potential for addiction are routinely prescribed for a variety of conditions which could be treated successfully with non-drug alternatives. As a result, prescription medications are a leading cause of death in the United States, killing more than 100,000 Americans annually and directly or indirectly generating numerous pesky lawsuits -- a growing headache for the industry. If doctors begin equating medication risks with rat poison, perhaps they'll exercise more caution when writing prescriptions, and stop dispensing pills like jelly beans. A subsequent decline in pharmaceutical sales and corresponding reduction in profits would be anticipated. However, some short-term monetary losses might be offset by future sales, based on the assumption that a certain percentage of patients not killed by drugs on the market today would become customers for future drugs entering the market tomorrow. This hypothesis has never been tested; data is lacking, and additional research is warranted. The universal adoption of a "rodenticide standard" will mark the dawn of a new medical era. By adopting rigorous new guidelines requiring every drug entering the market to be at least as safe and effective as rat poison, many lives will be saved, litigation avoided, and negative publicity minimized. No longer merely "better than nothing," medicine of the future would actually be superior to nonlethal quantities of a known toxic substance. The bar has been raised from sugar pill to rodenticide. Suggestions for further reading: Antonuccio, Burns and Danton, Antidepressants: a triumph of marketing over science?, Prevention & Treatment, 2002. de Craen et al., Effect of colour of drugs: systematic review of perceived effect of drugs and of their effectiveness, British Medical Journal, 1996. Heres et al., Why Olanzapine Beats Risperidone, Risperidone Beats Quetiapine, and Quetiapine Beats Olanzapine: An Exploratory Analysis of Head-to-Head Comparison Studies of Second-Generation Antipsychotics , American Journal of Psychiatry, 2006. Jackson, Grace E, Open Letter to the Federal Coordinating Council for Comparative Effectiveness Research , April 12, 2009. Lacasse and Leo, Serotonin and Depression: A Disconnect between the Advertisements and the Scientific Literature , PLoS Medicine, 2005. Lewis and Warlow, How to spot bias and other potential problems in randomised controlled trials, Journal of Neurology, Neurosurgery and Psychiatry, 2004. Melander et al., Evidence b(i)ased medicine--selective reporting from studies sponsored by pharmaceutical industry: review of studies in new applications, British Medical Journal, 2003. Moncrieff, Wessely, and Hardy, Active placebos versus antidepressants for depression, Cochrane Database of Systematic Reviews, 2004. Montori et al., Users' guide to detecting misleading claims in clinical research reports, British Medical Journal, 2004. Moynihan, Ray, Who pays for the pizza? Redefining the relationships between doctors and drug companies, British Medical Journal, 2003. Latest studies from the Bonkers Institute: * Schizophrenia Treatment in Seven Easy Steps © 2009 Bonkers Institute for Nearly Genuine Research
Bonkers Institute for Nearly Genuine Research
bonkersinstitute.org
* Addictive Properties of Shiitake Sesame Vinaigrette
* Utilization of Placebo Rat Poison in Controlled Clinical Trials
* Chemical Imbalance Not Otherwise Specified: Useful Diagnostic Category?
* Science Made Simple: Shopper's Guide to Mental Disorders
* Therapeutic Efficacy of Cash in the Treatment of Anxiety and Depressive Disorders
* Everything You Need to Know About Electroshock
Post Comment Private Reply Ignore Thread
Top • Page Up • Full Thread • Page Down • Bottom/Latest
Begin Trace Mode for Comment # 3.
#3. To: All (#0)
There are no replies to Comment # 3. End Trace Mode for Comment # 3.
Top • Page Up • Full Thread • Page Down • Bottom/Latest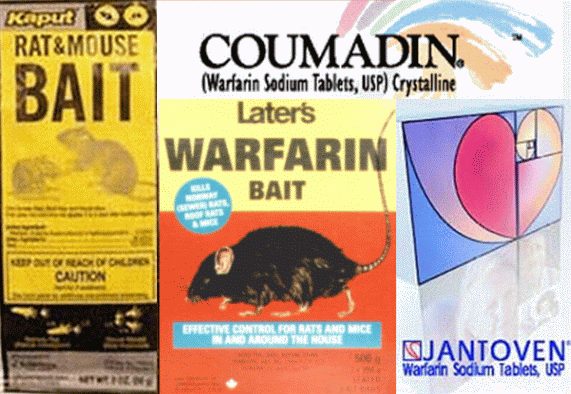
Replies to Comment # 3.